The research focuses of the Exercise Energetics Laboratory is how mitochondrial energy production in skeletal and cardiac muscle is precisely matched to energy demand, and how this coordination is altered during exercise, aging, and disease. Her work centers on mitochondrial function and energetics, with particular emphasis on how ATP production is regulated in muscle and heart tissue under physiological and pathological conditions.
Control of Mitochondrial Oxidative Phosphorylation
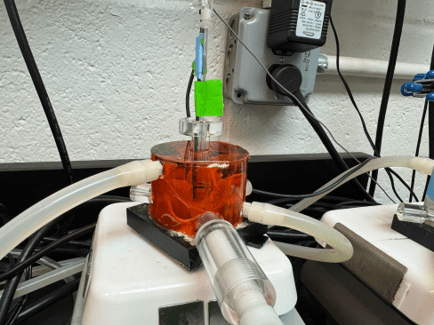
A major focus of the Exercise Energetics Laboratory is to determine how cardiac and skeletal muscle mitochondria adjust ATP production to match tissue energy demand. Our work seeks to reveal how ATP production is activated during an increase in muscle work. Work by our laboratory has shown that in cardiac mitochondria, ATP synthesis and transport are responsible for the majority of resistance during mitochondrial ATP production, and that calcium increases the effective activity of the entire ATP production pathway. Further, we have shown that cardiac ischemia blunts the ability of the mitochondria to produce ATP. Together, these data indicate that the activity of the components of the ATP production pathway are dynamically regulated, and our laboratory continues work to uncover how this dynamic regulation occurs and investigate strategies to enhance ATP synthesis.
Acute Regulation of Mitochondrial Supercomplexes
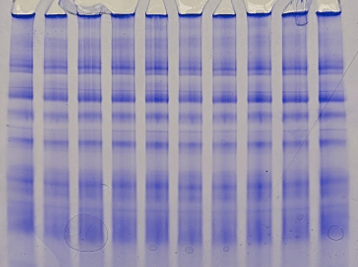
A second major focus of the Exercise Energetics Laboratory is to understand how mitochondrial structure and organization influence oxidative capacity in cardiac and skeletal muscle. Our work investigates how electron transport chain complexes interact to form supercomplexes and how these interactions regulate mitochondrial respiration. Because mitochondrial oxidative capacity is governed not only by mitochondrial content but also by the effective activity and organization of electron transport chain complexes, we are working to elucidate how physiological signals can alter protein–protein interactions within the electron transport chain, leading to changes in supercomplex formation and mitochondrial function. These findings highlight the role of mitochondrial electron transport chain organization in regulating ATP production and provide insight into mechanisms that contribute to energetic dysfunction with aging and disease.
Oxygenation and Mitochondrial Function in Whole Hearts
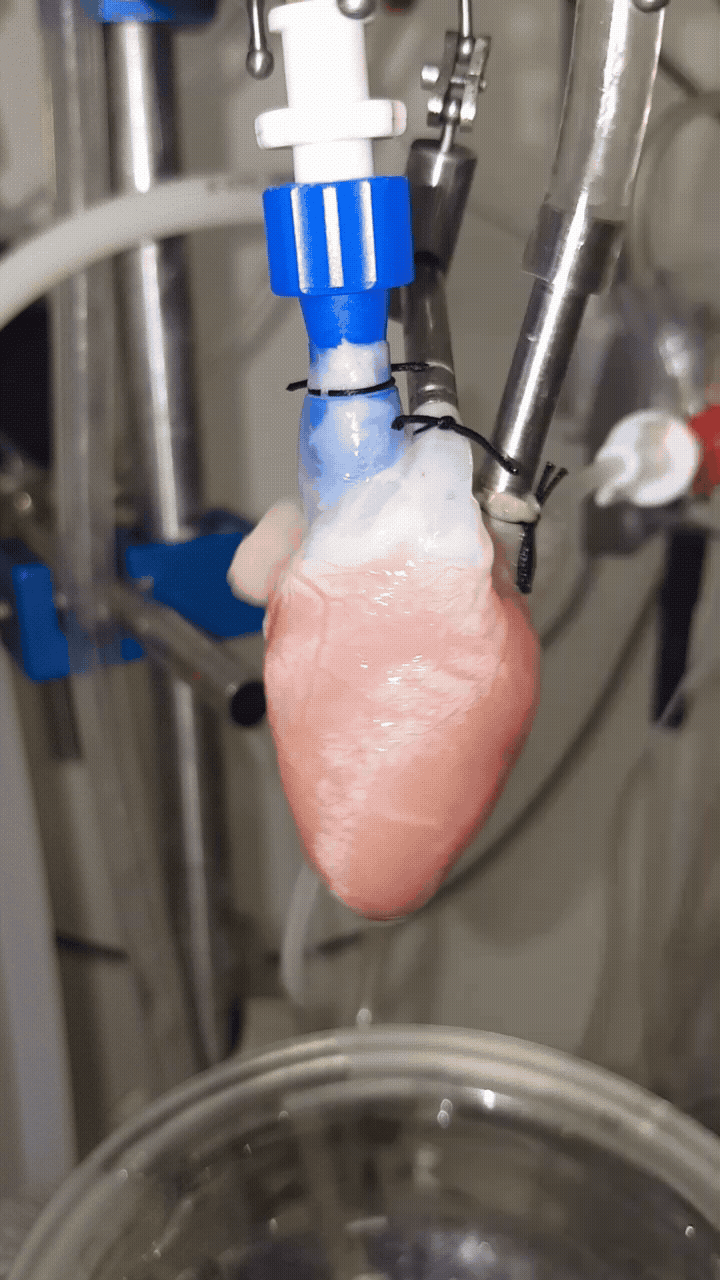
The laboratory also investigates the relationship between oxygen delivery and mitochondrial function using excised, perfused heart preparations and transmural optical spectroscopy to monitor myoglobin oxygenation and mitochondrial redox state in real time. These studies demonstrate that crystalloid-perfused hearts are oxygen limited at functional, cellular, and mitochondrial levels. Strategies to improve oxygenation include the use of oxygen-carrying perfluorocarbons and exogenous vasodilators, providing insight into the regulation of cardiac oxygenation and mitochondrial energetics. Further, this work has demonstrated that beta-adrenergic stimulation of heart rate differs between young and old, male and female hearts, indicating a role of estrogen in the regulation of cardiac function.