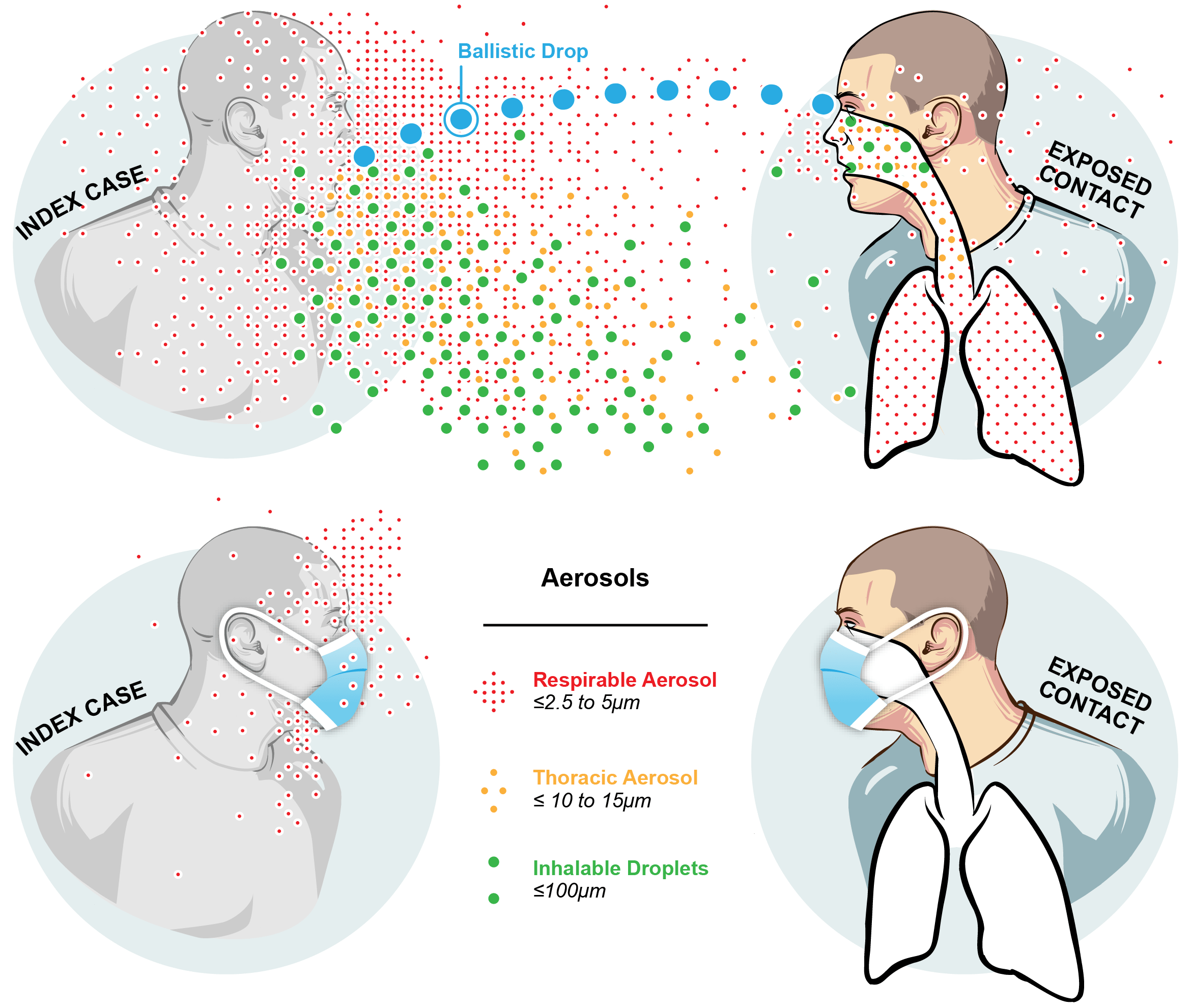
The Public Health Aerobiology Lab, led by Dr. Donald Milton, studies airborne infection transmission through multidisciplinary work including a virology lab, a bioaerosol lab and a clinic. Our lab is part of the Department of Global, Environmental, and Occupational Health. The research that we conduct is crucial in informing policy around environmental and occupational health.
Studying Airborne Infection Transmission

The laboratory supports studies of:
- airborne infection transmission
- influenza epidemiology
- bioaerosol exposure in asthma
- non-invasive monitoring of exhaled biomarkers
- the role of germicidal ultraviolet light (gUV) devices in disinfection of airborne pathogens
Capabilities of the lab include culturing viruses, RT-qPCR, analysis of exhaled breath particles, bioaerosol sampling and immunoassay.
Some of our current and former sponsors include:
PHAB Lab Team
Principal Investigators:
Bioaerosol Laboratory: Dr. Kristen Coleman, Dr. Jonathan Vyskocil, Phil Lubet, Maddie Sawyer, Anna Pulley,
Virology Laboratory: Dr. Sheldon Tai, Dr. Amrita Mandal, Alycia Smith
Research Clinic: Yi Esparza, Julie Corrigan, Dr. Barbara Albert, Zahra Iskandar, Saratu Kehinde
Communication Research: Dr. Kate McPhaul, Young Yun, Ning Xu
Project & Data Management: Dr. Filbert Hong, Isabel Sierra Maldonado, Sulakkhana De Saram
Epidemiology & Data Analytics: Dr. Donald Milton, Dr. Jianyu Lai, Jessica Xu
Post-Doctoral Fellows: Dr. Jianyu Lai, Dr. Jonathan Vyskocil, Dr. Amrita Mandal
Doctoral Students: Anna Pulley, Young Yun, Anamarie Leduc
Alumni: Dr. P. Jacob Bueno de Mesquita, Naja Fadul, Dr. Jennifer German, Aaron Kassman, Michael Lutchenkov, Molly Oertel, Vivek Ravichandran, Delwin Suraj, Rhonda Washington-Lewis, Dr. Somayeh Youssefi, Dr. Petri Kalliomaeki, Aditya Srikakulapu, Maria Schanz, Louie Gold, Catherine Nguyen, Ali Hassani, Will Smith, Olga Volchansky, Beata Assadi
Become an Undergraduate Research Assistant

We welcome undergraduate students seeking to gain research experience. Undergrad Research Assistants (URAs) can work with any of our specialized teams:
- Clinical Team
- Virology Team
- Bioaerosol Team
To join the PHAB Lab, you can contact us for an interview by filling out the interest form here. You will be required to enroll in ENVH 381 your first semester.
ENVH 381
(Waitlist for Fall 2025)
Students enrolled in ENVH 381 will receive credit for their work as URAs. They will require permission to enroll in the course. Once granted, they will enroll in the course on Testudo. Class will meet once a week (Fall 2025: Tuesdays at 4-4:50pm). They will also need to complete a minimum of 5 hours/week with their assigned team.
If interested, please fill out the interest form.
MIEH 309
Students enrolled in MIEH 309 will receive credit for their work as URAs. They will require permission to enroll in the course. Once granted, they will enroll in MIEH 309 Section 0204. They will need to complete their clinic/lab hours based on how many credits they enrolled for:
- 1 credit = 3 hrs/wk
- 2 credits = 5 hrs/wk
- 3 credits = 8-10 hrs/wk
Please note that the virology lab will require a minimum of 2 credits.
There's a waitlist for Fall 2025. If interested, please email phablab@umd.edu.
Do you want to do your Practicum/Capstone Project with us?
If you’re interested in completing your Practicum/Capstone Project with us, we require that you spend at least one semester working with us as a URA enrolled in ENVH 381 previous to doing your project. For your project, you will be assigned a supervisor to work with you. You will need to complete your Practicum/Capstone required hours separate from your class hours.
Dr. Don Milton Publications
ORCID: https://orcid.org/0000-0002-0550-7834
Google Scholar: https://scholar.google.com/citations?
Dr. Kristen Coleman Publications
ORCID: https://orcid.org/0000-0003-0024-3400
Google Scholar: https://scholar.google.com/citations?user=v0mVFaYAAAAJ
Dr. Kate McPhaul
ORCID: https://orcid.org/0000-0002-7008-142X
Google Scholar: https://scholar.google.com/citations?user=Epyr0xMAAAAJ&hl=en
Key Publications from the Public Health Aerobiology Laboratory
Nan Zhang, Yong Guo, Benjamin J. Cowling, Weiwei Huang, Wei Jia, Ao Li, Danting Luo, Donald K. Milton, Shengqi Wang, Hui-Ling Yen, Yinping Zhang, Yingxin Zhu, Hua Qian, Yuguo Li. Explosive household spread of the SARS-CoV-2 Omicron variant in China in late 2022. Building and Environment, Volume 256, 2024.
Lai J, Coleman KK, Tai S-H, German J, Hong F, Albert B, Esparza Y, Srikakulapu A, Schanz M, Sierra Maldonado I, Oertel M, Fadul N, Gold TL, Weston S, Mullins K, McPhaul KM, Frieman M, Milton DK. Exhaled Breath Aerosol Shedding of Highly Transmissible Versus Prior Severe Acute Respiratory Syndrome Coronavirus 2 Variants, Clinical Infectious Diseases (2022), ciac846.
Lai J, German J, Hong F, Tai S-H, McPhaul K, Milton DK, University of Maryland StopCOVID Research Group. Comparison of Saliva and Midturbinate Swabs for Detection of SARS-CoV-2, Microbiology Spectrum (2022).
Coleman KK, Tay DJW, Tan KS, Ong SWX, Than TS, Koh MH, Chin YQ, Nasir H, Mak TM, Chu JJH, Milton DK, Chow VTK, Tambyah PA, Chen M, Tham KW. Viral Load of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in Respiratory Aerosols Emitted by Patients With Coronavirus Disease 2019 (COVID-19) While Breathing, Talking, and Singing. Clin Infect Dis. 2022 May 30;74(10):1722-1728.
Adenaiye O, Lai J, Bueno de Mesquita PJ, Hong F, Youssefi S, German J, Tai S-H, Albert B, Schanz M, Weston S, Hang J, Fung C, Chung HK, Coleman KK, Sapoval N, Treangen T, Maljkovic Berry I, Mullins K, Frieman M, Ma T, Milton DK, University of Maryland StopCOVID Research Group. Infectious Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in Exhaled Aerosols and Efficacy of Masks During Early Mild Infection, Clinical Infectious Diseases (2021), ciab797.
de Assis, R.R., Jain, A., Nakajima, R. et al. Analysis of SARS-CoV-2 antibodies in COVID-19 convalescent blood using a coronavirus antigen microarray. Nat Commun 12, 6 (2021).
Prather K, Marr L, Schooley R, McDiarmid M, Wilson M, Milton DK. Airborne Transmission of SARS-CoV-2, Science 05 Oct 2020
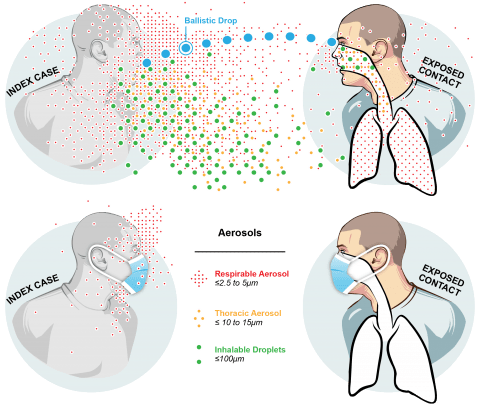
Milton DK. A Rosetta Stone for Understanding Infectious Drops and Aerosols. J Pediatric Infect Dis Soc 2020
(accepted manuscript here if you cannot access journal webpage)
Morawska L, Milton DK. It is Time to Address Airborne Transmission of COVID-19. Clin Infect Dis 2020
Bueno de Mesquita PJ, Noakes CJ, Milton DK. Quantitative aerobiologic analysis of an influenza human challenge-transmission trial. Indoor Air 2020
Bueno de Mesquita PJ, Nguyen-Van-Tam J, Killingley B, et al. Influenza A (H3) illness and viral aerosol shedding from symptomatic naturally infected and experimentally infected cases. Influenza Other Respir Viruses 2020; irv.12790.
Chia PY, Coleman KK, Tan YK, et al. Detection of air and surface contamination by SARS-CoV-2 in hospital rooms of infected patients. Nature Communications 2020;11(1):2800.
Morawska L, Tang JW, Bahnfleth W, et al. How can airborne transmission of COVID-19 indoors be minimised? Environ Int 2020;142:105832.
Nguyen-Van-Tam JS, Killingley B, Enstone J, et al. Minimal transmission in an influenza A (H3N2) human challenge-transmission model within a controlled exposure environment. PLoS Pathog 2020;16(7):e1008704.
Leung NHL, Chu DKW, Shiu EYC, et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nature Medicine 2020;1–5.
Zhu S, Jenkins S, Addo K, et al. Ventilation and laboratory confirmed acute respiratory infection (ARI) rates in college residence halls in College Park, Maryland. Environment International 2020;137:105537.
Fennelly KP, Acuna-Villaorduna C, Jones-Lopez E, Lindsley WG, Milton D. Microbial Aerosols: New Diagnostic Specimens for Pulmonary Infections. CHEST [Internet] 2019 [cited 2019 Nov 20];0(0).
(19)34113-3/abstract
Yan J, Grantham M, Pantelic J, et al. Infectious virus in exhaled breath of symptomatic seasonal influenza cases from a college community. Proc Natl Acad Sci USA 2018;115(5):1081–6.
Recent News Coverage of UMD StopCOVID Team Members
September 7, 2021 Maryland Institute of Environmental Health 688 Seminar:
Results of the UMD StopCOVID Study and High Efficiency Air Sanitation with Germicidal UV Light for Schools and Conference Rooms, presented by Jianyu Lai, MPH and Don Milton, MD, DrPH
View the zoom recording for MIEH 688 Seminar September 7, 2021.
Passcode: !+h8%j?Q
Infectious SARS-CoV-2 in Exhaled Aerosols and Efficacy of Masks During Early Mild Infection: We have analyzed the results of 13 months of the StopCOVID study. The manuscript is available at medRxiv.org [https://medrxiv.org/cgi/content/short/2021.08.13.21261989v1], and is undergoing review for publication.
Key points:
- Cases exhale infectious viral aerosols.
- SARS-CoV-2 is evolving toward more efficient airborne transmission.
- Loose-fitting masks significantly but moderately reduce viral RNA aerosol.
- Tight-fitting masks or respirators and ventilation/air cleaning are essential for worker protection in public-facing or crowded indoor workplaces.
Please also check out the article at Science News that talks about these results: https://www.sciencenews.org/article/covid-coronavirus-aerosol-droplets-airborne-evolution
Here's Why Mouthwash Is Not Going To Save You From Coronavirus. CNN.com (November 17, 2020).
UMD Scientists Say Vice Presidential Debate Needs Air Filtration System Due To Coronavirus. CBS Baltimore, October 7, 2020.
Beyond Plexiglass: Scientists Say This Simple Solution Could Keep VP Debate Safer. NPR, October 7, 2020.
The plexiglass barriers at tonight’s debate will be pretty useless, virus experts say. New York Times, October 7, 2020.
Answering COVID's Big Questions on Campus. Maryland Today, October 6, 2020.
The Flu May Linger in the Air, Just Like the Coronavirus. The New York Times, July 14, 2020.
We Need to Talk About Ventilation. Tufekci Z. The Atlantic. July 30, 2020.
Recent Lectures by Members of the UMD StopCOVID Team
Virtual Press Conference on COVID-19 Science Letter October 5, 2020
Dr. Milton's lecture for MIEH 688: Infectious Drops and Aerosols September 22, 2020
University of California San Francisco Medical Grand Rounds July 16, 2020: Dr. Milton
A Conversation: What Do Science and Data Say About the Near Term Future of Singing May 5, 2020 Webinar
Air Disinfection with Germicidal UV

Germicidal Ultraviolet (GUV) air disinfection is a technology that has been used for decades, and it’s safe and effective in inactivating viruses and other pathogens in the air. While ventilation and filtration are widely recommended to reduce risk of inhalation transmission, these interventions on their own cannot stop superspreading events. GUV air disinfection holds the promise of being able to stop these events, especially when layered with other preventative measures. Widespread adoption of GUV would allow us to pursue normal social and economic activities.
With the support of the Balvi Foundation, we have several projects that tackle some of the obstacles encountered for widespread adoption
Public Attitudes and Communications Research
A critical component essential to widespread GUV implementation is public understanding and acceptance of the technology. This project aims to conduct a number of focus groups with different audiences, that will then inform clearer messaging and communication strategies around the use of GUV. With this project, we’ll also develop a GUV Information Hub that will be accessible to the public.
Training and Certification of Technicians and Contractors
A major barrier of implementation is a lack of trained workforce. Funding for this program has facilitated the creation of The GUV Workforce Training Program, which entailed the design of course materials through expert consultation and training of trainers for educating and certifying professionals in the US and abroad in safe planning, design, implementation, and maintenance of GUV in public spaces. We have hosted three of the training sessions here in SPH within the last year and a half, and have also developed an apprenticeship level program that will be available to access online for free.
Optimization of Far-GUV Installation Designs
The GUV-optimization study aims to address some of the obstacles preventing its widespread use by identifying the best far-GUV installation designs. Lab experiments will aid in this process by exposing bacteriophages and influenza virus to different UV doses, and using those results to inform designs in a field trial to demonstrate real-world effectiveness of 222 nm GUV in inactivating viruses and other pathogens. This will also allow further inspection on the effect of GUV on indoor air chemistry. Furthermore, there are plans to conduct RCTs to provide evidence of its effectiveness.
Evaluating Modes of Influenza Transmission (EMIT-2)
Sponsor: National Institute of Allergy and Infectious Diseases
Participating Institutions/Departments: UMD SPH, UMD Clark School of Engineering, UMD CBMG; UMSOM in Baltimore; University of Hong Kong School of Public Health; Icahn School of Medicine at Mt. Sinai; Global Health Institute at the University of Wisconsin-Madison; University of Michigan School of Public Health; Aerosol Dynamics
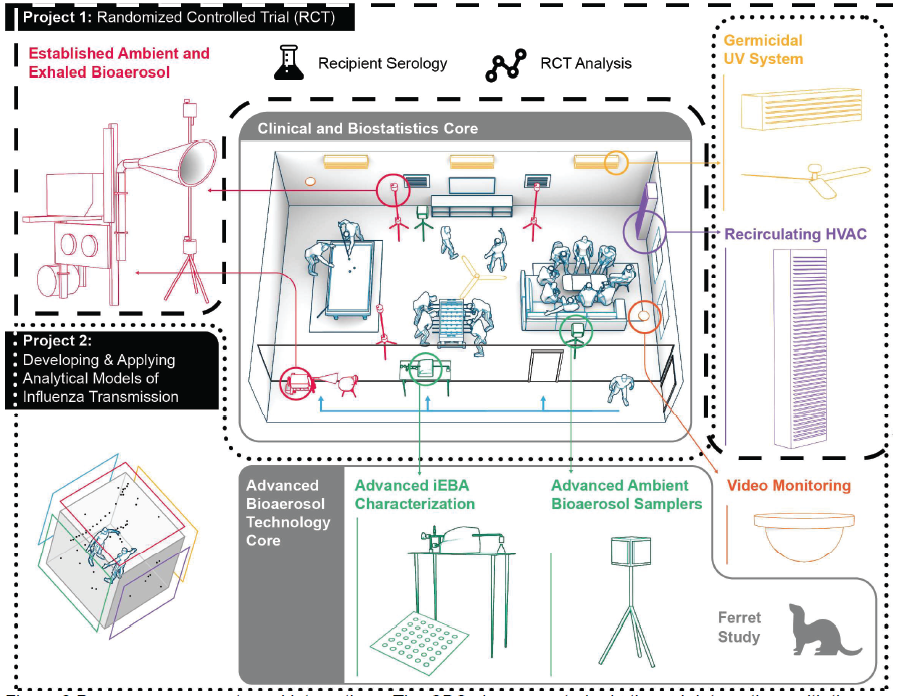
Project 1 (Evaluating Modes of Influenza Transmission using a Randomized Controlled Trial, EMIT-2-RCT)
Lead Investigator: Don Milton, UMD SPH
Co-Investigators: Ben Cowling, HKU SPH; Aubree Gordon, UMich SPH; Florian Krammer, Mt. Sinai School of Medicine
- Identify the dominant mode of transmission using naturally infected influenza donors in an RCT of air sanitation-ventilation and hand hygiene-face shield interventions.
- Determine the impact of aerosol exposure on disease severity.
- Investigate the impact of serologic and mucosal antibody levels on influenza transmission, susceptibility and immunologic response to infection.
Project 2 (Developing and Applying Analytical Models of Influenza Transmission)
Lead Investigator: Jelena Srebric, UMD ENG
Co-Investigators: Aubree Gordon, UMich SPH; Gabriele Neumann, UWis GHI
- Support the cohort study experimental setup: face shield, ventilation, UR-GUV application
- Develop two analytical models for source characterization:
- A high-fidelity model to identify variability in risk due to temporal and spatial distributions of virus concentration.
- A well-mixed model (for well-mixed conditions) using CO2 as a stand-in to assess viral aerosol exposure and dose. - Apply the analytical models to house cohort & ferret studies
Clinical and Biostatistics Core (CBC)
Lead Investigator: Kate McPhaul, UMD SPH
Co-Investigators: Wilbur Chen, Justin Ortiz, Shuo Chen, UMSOM
- Provide regulatory and safety infrastructure for a randomized controlled trial of influenza transmission.
- Screen for health adult volunteers (Recipients), willing to be exposed to community acquired influenza during the influenza season, and index cases with acute influenza infection (Donors).
- Expose un-infected eligible volunteers (Recipients) to influenza-infected index case patients (Donors).
Advanced Bioaerosol Technology Core (ABTC)
Lead Investigator: Don DeVoe, UMD ENG
Co-Investigators: Meg Scull, Gregg Duncan, UMD CMBG; Arantza Eiguren-Fernandez, Greg Lewis, Aerosol Dynamics; Yoshihiro Kawaoka, Gabriele Neumann, UWis GHI
- Develop a compact platform for ambient sampling and culture.
- Develop an instrument for efficient exhaled breath sampling.
- Develop a hydrogel collection target and an optimized cell line for enhanced infectivity analysis.
- Develop a digital culture microarray to study distribution of viable virus in aerosols.
Center for Research on Influenza Pathogenesis and Transmission (CRIPT) @ UMD

Human-to-Human Transmission Studies (aka the GotFluToo Study)
Part of the NIAID Centers of Excellence for Influenza Research and Response (CEIRR)
Sponsor: National Institute of Allergy and Infectious Diseases (NIAID)
Principal Investigator: Don Milton (UMD SPH)
Co-Investigators: Jelena Srebric (UMD ENG), Charles Ma (UMD SPH)
Objectives:
- Identify acute respiratory infection (ARISs) outbreaks and describe environmental factors that facilitate transmission; and
- Test the hypothesis that building ventilation at <5 liters per second per person is associated with high risk of ARIs like influenza and SARS-CoV-2
This research will provide evidence of the effectiveness of ventilation as a control measure for ARIs, which is especially relevant in preventing and preparing for outbreaks and pandemics since vaccines and other medical interventions take time to develop
- The target population is the UMD and surrounding community within walking distance of campus who share a bedroom but not bed with one or more roommates.
- The goal is to enroll up to 1,000 roommate pairs (2,000 participants) over the course of 4 years.
- The following items are collected from individuals:
- Online consent
- Questionnaires
- Biological specimens (nasal swabs, saliva, blood)
- Environmental data (CO2, PM2.5, air temperature, etc.)
- Exhaled breath samples
- Participants will receive compensation for their time.
StopCOVID (2020-2023)
Publications:
Lai J, Coleman KK, Tai SS, German J, Hong F, Albert B, Esparza Y, Rastogi D, Srikakulapu A, Kalliomäki P, Schanz M, Smith AA, Sierra Maldonado I, Oertel M, Fadul N, Gold TL, McPhaul K, Ma T, Cowling BJ, Milton DK. Relative efficacy of masks and respirators as source control for viral aerosol shedding from people infected with SARS-CoV-2: a controlled human exhaled breath aerosol experimental study. EBioMedicine. 2024 Jun;104:105157. https://doi.org/10.1016/j.ebiom.2024.105157
Lai J, Coleman KK, Tai SH, German J, Hong F, Albert B, Esparza Y, Srikakulapu A, Schanz M, Sierra Maldonado I, Oertel M, Fadul N, Gold TL, Weston S, McPhaul K, Frieman M, Milton DK. Exhaled Breath Aerosol Shedding by Highly Transmissible Versus Prior SARS-CoV-2 Variants. Clinical Infectious Diseases (2022), ciac846, https://doi.org/10.1093/cid/ciac846
Lai J, German J, Hong F, Tai S-H, McPhaul K, Milton DK, University of Maryland StopCOVID Research Group. Comparison of Saliva and Midturbinate Swabs for Detection of SARS-CoV-2. Microbiology Spectrum (2022), https://doi.org/10.1128/spectrum.00128-22
Adenaiye O, Lai J, Bueno de Mesquita PJ, Hong F, Youssefi S, German J, Tai S-H, Albert B, Schanz M, Weston S, Hang J, Fung C, Chung HK, Coleman KK, Sapoval N, Treangen T, Maljkovic Berry I, Mullins K, Frieman M, Ma T, Milton DK, University of Maryland StopCOVID Research Group. Infectious Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in Exhaled Aerosols and Efficacy of Masks During Early Mild Infection, Clinical Infectious Diseases (2021), ciab797, https://doi.org/10.1093/cid/ciab797
Adenaiye O, Bueno de Mesquita PJ, Wu Q, Hong F, Lai J, Chen S, Milton DK; Prometheus@UMD Consortium. The effect of COVID-19 stay-at-home order and campus closure on the prevalence of acute respiratory infection symptoms in college campus cohorts. Influenza Other Respi Viruses (2021); 15: 331-335. https://doi.org/10.1111/irv.12837
Objectives:
Find out how people transmit COVID-19 and how to prevent transmission:
- How much airborne virus does an infected person exhale?
- How much virus is released into the air when an infected person breathes, talks, or sings?
- How well do surgical and homemade masks block release of airborne virus?
Another goal is to gather samples that can be used to better understand how the body fights the infection.
Sponsors:
Prometheus@UMD (2017-2020)
Publications:
Xiao J, de Mesquita JB, Leung NHL, Adenaiye O, Tai S, Frieman MB, Hong F, Chu DKW, Ip DKM, Cowling BJ, Milton DK; Prometheus-UMD Consortium. Viral RNA and infectious influenza virus on mobile phones of influenza patients in Hong Kong and the United States. J Infect Dis. (2021 Sep 17):jiab464. https://doi.org/10.1093/infdis/jiab464. PMID: 3453432
Adenaiye O, Bueno de Mesquita PJ, Wu Q, Hong F, Lai J, Chen S, Milton DK; Prometheus@UMD Consortium. The effect of COVID-19 stay-at-home order and campus closure on the prevalence of acute respiratory infection symptoms in college campus cohorts. Influenza Other Respi Viruses (2021); 15: 331-335. https://doi.org/10.1111/irv.12837
de Assis RR, Jain A, Nakajima R, Jasinskas, A, Felgner J Obiero JM, Norris PJ, Stone M, Simmons G, Bagri A, Irsch J, Schreiber M, Buser A, Holbro A, Battegay M, Hosmier P, Noesen C, Adenaiye O, Tai S, Hong F, Milton DK, Davies DH, Contestbable P, Corash LM, Busch MP, Felgner PL, Khan S. (2021) Analysis of SARS-CoV-2 Antibodies in COVID-19 Convalescent Blood Using A Coronavirus Antigen Microarray, Nature Communications 12, 6. https://doi.org/10.1038/s41467-020-20095-2
Zhu S, Jenkins S, Addo K, Romo S, Layne A, Ehizibolo J, Dalgo D, Matisse N, Hong F, Adenaiye OO, Bueno de Mesquita PJ, Albert BJ, Washington-Lewis R, German J, Tai S, Youssefi S, Milton DK, Srebric J. (2020) Ventilation and laboratory confirmed acute respiratory infection (ARI) rates in college residence halls in College Park, Maryland, Environment International 137: 105537. https://doi.org/10.1016/j.envint.2020.105537
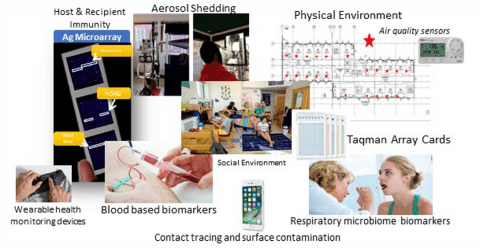
Objectives:
- Identify and characterize contagious phenotype of acute respiratory infection (ARI)
- Identify biomarkers of infection and contagiousness
- Test wearable sensors for early detection of ARI & outbreaks
- Collect PBMCs for identification of epigenetic markers of infection
- Characterize the role of aerosols and built environment in ARI transmission
Sponsors:
- Defense Advanced Research Projects Agency (DARPA) Biological Technologies Office
- Biomedical Advanced Research Development Authority (BARDA) ENACT: https://drive.hhs.gov/enact.html
Website (historical): https://catch.umd.edu
Below we show some live data collected on our participant's engagement and compensation.